
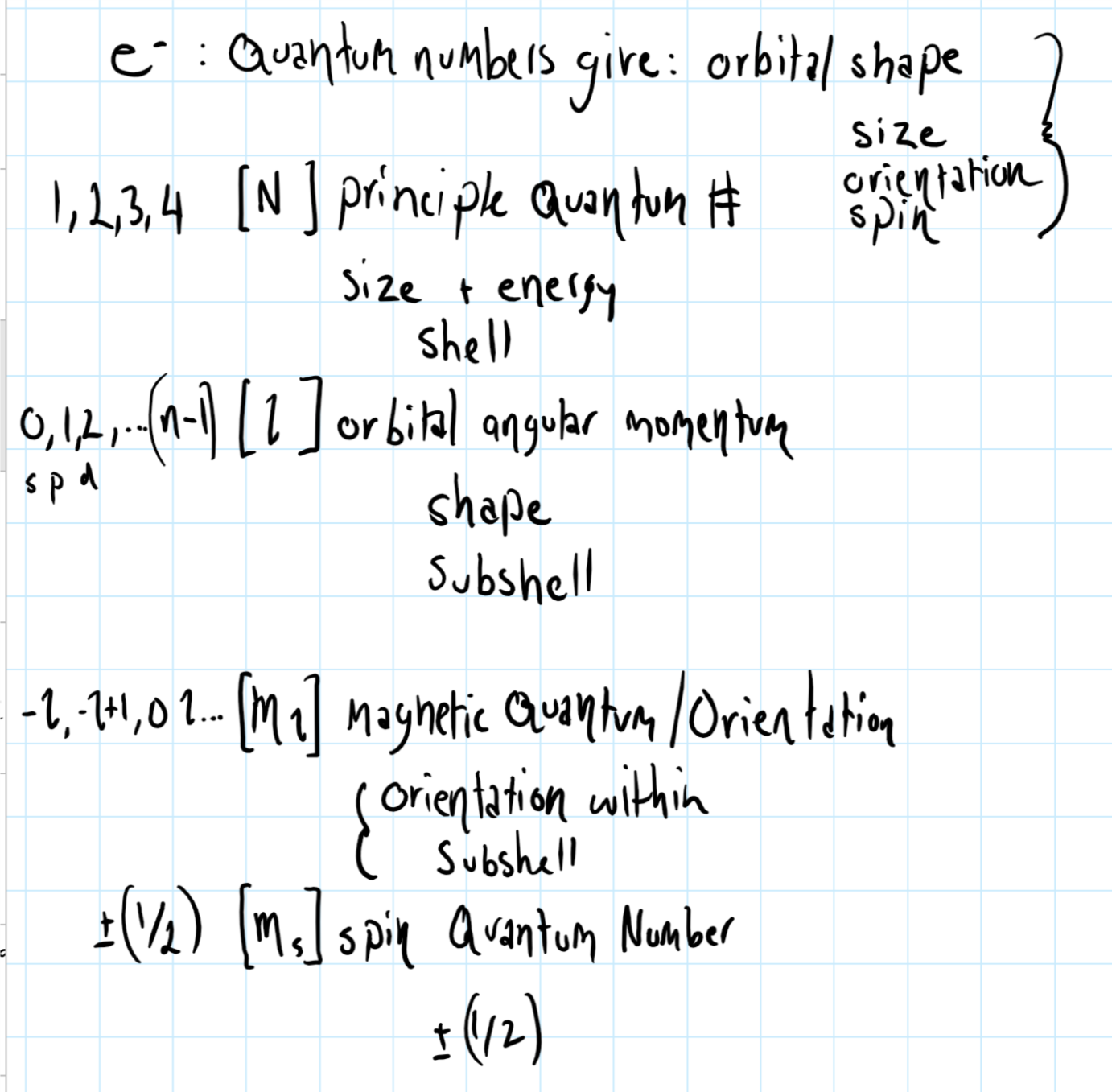

Determine whether the molecule or molecular ion is homonuclear or heteronuclear. Therefore, in the electronic configuration of Chromium and Copper, if one electron of 4 s 2 comes to the previous shell, it will make 3 d 4 to 3 d 5 and 3 d 9 to 3 d 10. It assumes that you know about simple atomic orbitals - at least as far as the way they are named, and their relative energies. For the Cu2+ ion we remove a total of two electrons (one from the 4s1 and one form the 3d10) leaving us with.
4p orbital quantum numbers free#
Consider a Ni(II) complex, electronic configuration is d8 For a free metal ion, S = 1, L = 3 and μ= √L(L+1) + 4S(S+1) = 4. Co 2+ is d 7 and these are all weak … Jan 13, 2014 There are 27 electrons for the Cobalt a The s,p,d,f configuration for cobalt (Co) is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^7#, determined by the position of the element on the periodic table. However, there are few reports on modulating the electronic configuration of partial Co Oct 3+ (t 6 2g e 0 g) to Co Oct 2+ (t 6 2g e 1 g) for further promoting their OER … Apr 19, 2019 Asymmetric diatomic molecules and ions such as CO, NO, and NO + also have the ordering of energy levels shown on the left because of sp mixing. For example, the electronic configuration for the element boron can be writen as: 1s 2 2s 2 2p 1. For the Cobalt it is a bit more difficult, like you write the configuration you cannot find Solution. The attached image shows the Electronic Configuration of Co +2 and Co +3 ions in the ground state. 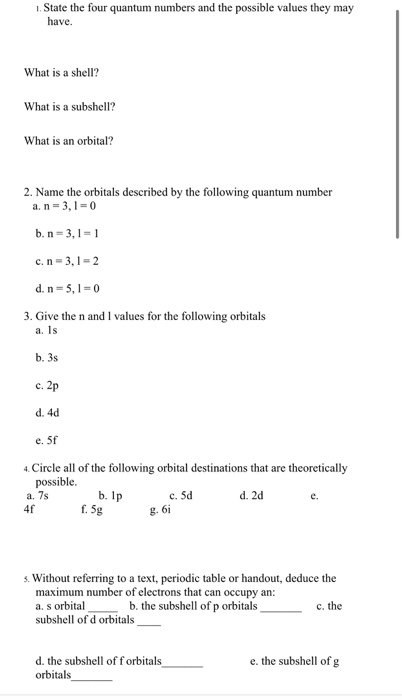
"Co" is in Period 4 of the Periodic Table, and "Ar" is the preceding noble gas.


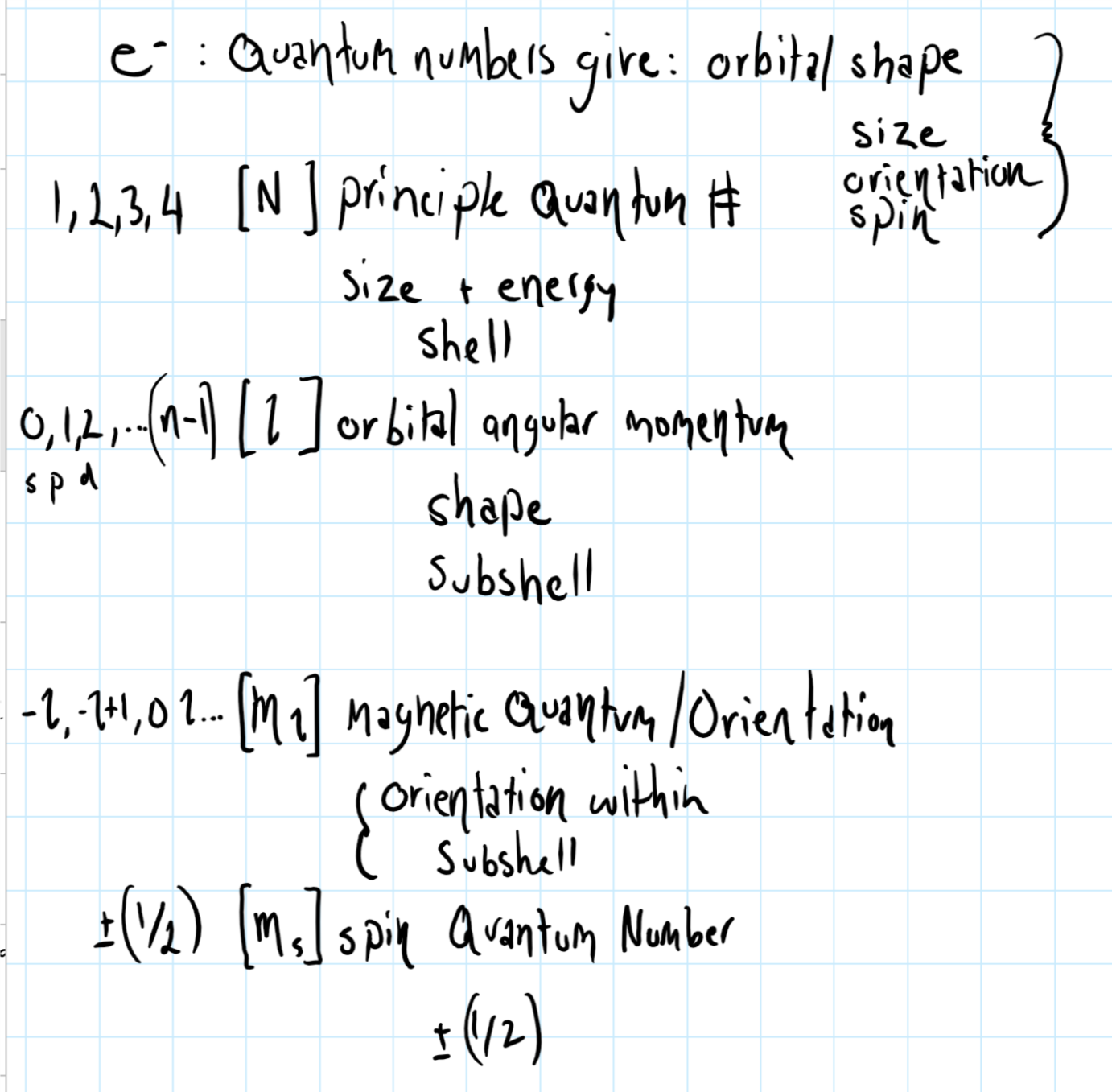




 0 kommentar(er)
0 kommentar(er)
